Medical device titanium coils require specialized packaging to ensure sterility, protection, and functionality. The right custom packaging solution safeguards these sensitive components and maintains their performance from manufacturing to the point of use.
Titanium coils are integral components in various medical devices, offering biocompatibility, strength, and flexibility. However, these properties also make them susceptible to damage during handling, transportation, and storage. Selecting the appropriate custom packaging is paramount for maintaining the integrity and sterility of titanium coils used in critical medical applications.
Understanding the Packaging Needs of Titanium Coils
Several factors must be considered when designing custom packaging for medical device titanium coils:
- Sterility: Maintaining sterility is non-negotiable. The selected materials and packaging processes must prevent microbial contamination throughout the product’s lifecycle. Processes like ethylene oxide (EtO) sterilization, gamma irradiation, or e-beam sterilization may be required.
- Physical Protection: Coils are vulnerable to bending, kinking, and other forms of physical damage that can compromise their functionality. The packaging must offer adequate protection against these hazards.
- Material Compatibility: The packaging materials must not react with the titanium coil, leach harmful substances, or degrade over time.
- Ease of Use: The packaging should allow healthcare professionals to easily access the titanium coil without compromising sterility or damaging the device.
- Regulatory Compliance: Medical device packaging must comply with stringent regulatory requirements, including ISO standards and FDA guidelines.
Custom Packaging Solutions for Titanium Coils
Several custom packaging options are available for medical device titanium coils, each offering unique advantages and disadvantages:

- Rigid Trays: Rigid trays, often made of thermoformed plastic, provide excellent physical protection and can be designed with custom features to secure the coil in place.
- Pouches: Flexible pouches offer a cost-effective solution for sterile packaging. They can be made from various materials, including Tyvek®, films, and laminates.
- Blister Packs: Blister packs offer a clear view of the titanium coil and provide good protection against contamination.
- Hybrid Solutions: Combining rigid and flexible packaging elements can provide the optimal balance of protection, sterility, and cost-effectiveness. For instance, a rigid tray may be sealed with a Tyvek® lid.
Materials for Medical Device Packaging
The selection of appropriate packaging materials is crucial for ensuring the safety and efficacy of medical device titanium coils. Some commonly used materials include:
- Tyvek®: This spunbonded olefin material is known for its exceptional microbial barrier properties, tear strength, puncture resistance, and compatibility with various sterilization methods.
- Medical-Grade Films: Polyethylene (PE), polypropylene (PP), and polyester (PET) films are often used in flexible packaging applications due to their barrier properties, chemical resistance, and cost-effectiveness.
- Thermoformable Plastics: Polyvinyl chloride (PVC), polystyrene (PS), and polyethylene terephthalate (PET) are used for rigid trays and blister packs, offering excellent protection and customization options.
The Custom Packaging Design Process
Developing a custom packaging solution for medical device titanium coils typically involves the following steps:
- Requirements Assessment: The first step is to thoroughly assess the requirements of the titanium coil, including its dimensions, weight, fragility, sterilization method, and intended use.
- Material Selection: Based on the requirements assessment, appropriate packaging materials are selected, considering their properties, cost, and compatibility with the sterilization process.
- Design Development: Packaging engineers create detailed drawings and prototypes of the custom packaging solution, incorporating features to protect the coil and ensure ease of use.
- Testing and Validation: The packaging is subjected to rigorous testing to ensure it meets the required performance standards, including sterility, strength, and barrier properties.
- Manufacturing and Assembly: Once the packaging design is validated, it is manufactured and assembled in a cleanroom environment to maintain sterility.
Working with a Medical Device Packaging Expert
Collaborating with an experienced medical device packaging company can streamline the development process and ensure a successful outcome. Look for a partner with:
- Expertise in Medical Device Packaging: A deep understanding of the regulatory requirements, material options, and design principles specific to medical device packaging.
- Cleanroom Manufacturing Facilities: The ability to manufacture and assemble packaging in a controlled environment to maintain sterility.
- Testing and Validation Capabilities: In-house testing facilities to perform a range of validation tests, ensuring that the packaging meets the required performance standards.
- Collaborative Approach: A willingness to work closely with you to design a packaging solution that meets your specific needs and budget.
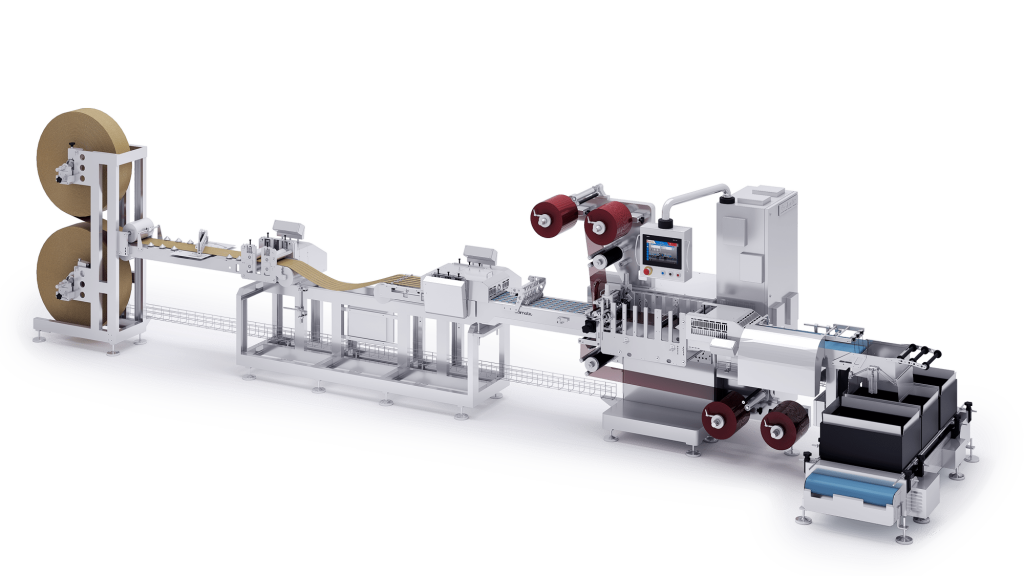
The Role of Sterile Packaging Machines
Sterile packaging machines are essential for ensuring the integrity of medical device packaging. These machines automate the packaging process, minimizing human contact and reducing the risk of contamination. They are often used in conjunction with cleanroom environments to create a sterile packaging environment.
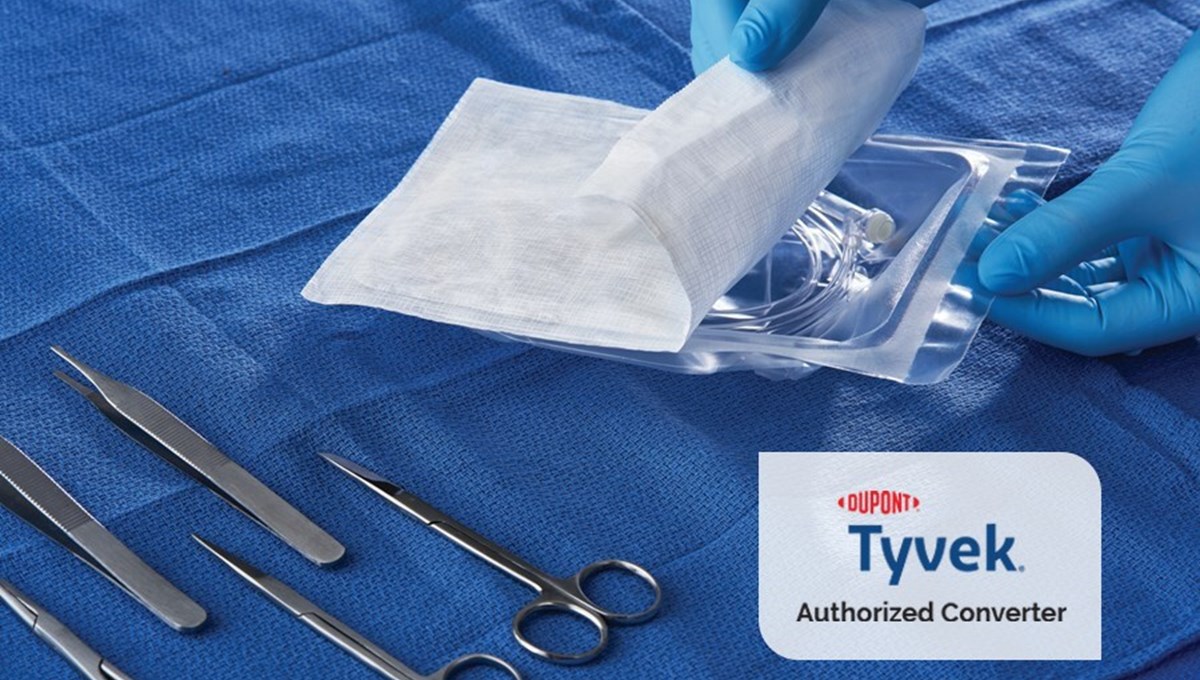
Sterile packaging machines are designed to create a secure and sterile seal, crucial for maintaining the integrity of the medical device until it is ready for use. These machines contribute to the overall quality and safety of the final product.
Data Comparison for Medical Coil Materials
| Here is a comparison of common materials used in medical coil manufacturing regarding tensile strength and biocompatibility: | Material | Tensile Strength (MPa) | Biocompatibility | Description |
|---|---|---|---|---|
| 304V Stainless Steel | 515 – 620 | Good | Economical choice with good strength; corrosion resistance can be enhanced. | |
| Nitinol | 895 | Excellent | Shape memory properties and superelasticity; more expensive. | |
| Titanium (Grade 5) | 880 – 950 | Excellent | High strength-to-weight ratio; non-magnetic; expensive. | |
| Platinum-Iridium (PtIr) | 380 | Excellent | Radiopaque, often used for markers; very expensive. |
Medical Wire Coiling Specifications for Titanium Coils
For titanium coils, the following specifications are typically considered:
- Wire Diameter: 0.001″ to 0.032″
- Coil OD: 0.010″ to 0.75″
- Coil Length: Up to 96″ (application dependent)
- Materials: Titanium (various grades).
Examples of Medical Devices Using Titanium Coils
Titanium coils are used in a wide range of medical devices, including:
- Catheters: Reinforcement coils for improved flexibility and pushability.
- Guidewires: Providing torque control and steerability.
- Stents: Delivering radial support and scaffolding.
- Electrophysiology Catheters: Facilitating signal transmission and ablation.
Choosing the right custom packaging for medical device titanium coils is an investment in product quality, patient safety, and regulatory compliance. By carefully considering the requirements of the coil and working with a trusted packaging partner, manufacturers can ensure their products arrive in pristine condition and perform as intended.











